- MathNotebook
- MathConcepts
- StudyMath
- Geometry
- Logic
- Bott periodicity
- CategoryTheory
- FieldWithOneElement
- MathDiscovery
- Math Connections
Epistemology
- m a t h 4 w i s d o m - g m a i l
- +370 607 27 665
- My work is in the Public Domain for all to share freely.
- 读物 书 影片 维基百科
Introduction E9F5FC
Questions FFFFC0
Software
Collect ways of figuring thing out chemistry
化学
Readings
 History of chemistry
History of chemistry
 Analytical chemistry
Analytical chemistry
 History of thermodynamics
History of thermodynamics
 Chemistry
Chemistry
- MIT: Introduction to Experimental Chemistry
- List of unsolved problems in chemistry
- Stanford: Philosophy of Chemistry
 Philosophy of chemistry
Philosophy of chemistry
- Chemical equipment
Books
- Bernard Jaffe. Crucibles: The Story of Chemistry from Ancient Alchemy to Nuclear Fission.
- Jonathan Norton. Crusaders of chemistry; six makers of the modern world
- Derek B Lowe. The Chemistry Book: From Gunpowder to Graphene, 250 Milestones in the History of Chemistry
- The Alchemy of Air
- The development of modern chemistry by Aaron J Idhe
- Buckingham, 'Chasing the Molecule'
- Transforming Matter: A History of Chemistry from Alchemy to the Buckyball.
Videos
- BBC Series: 'Chemistry: A Volatile History', available on Youtube, 3 one hour parts
- 25 Chemistry Experiments in 15 Minutes
- Peter Morris: The history of chemical laboratories, 1600-2000
Ways of figuring things out
General theme: Responsibility for agency.
Initial
Collecting
- The earliest recorded metal employed by humans seems to be gold, which can be found free or "native". Small amounts of natural gold have been found in Spanish caves used during the late Paleolithic period, around 40,000 BC.
- Silver, copper, tin and meteoric iron can also be found native.
- In the search for the philosophers stone, Justus von Liebig states that 'it was indispensable that every substance accessible... should be observed and examined'.
- Cronstedt discovered the mineral scheelite in 1751, which he named tungsten, meaning "heavy stone" in Swedish.
Old materials
Seeking first principles
- Greek philosophers had debated which substance was the arche ("first principle"), or primordial element from which everything else was made; Heraclitus championed fire, Thales supported water, and Anaximenes plumped for air. Anaximander argued that the primordial substance was not any of the known substances, but could be transformed into them, and they into each other. Empedocles was the first to propose four elements, fire, earth, air, and water. He called them the four “roots” (ῥιζώματα, rhizōmata).
- Boyle appealed to chemists to experiment and asserted that experiments denied the limiting of chemical elements to only the classic four: earth, fire, air, and water.
- Elementary Treatise of Chemistry. This text clarified the concept of an element as a substance that could not be broken down by any known method of chemical analysis and presented Lavoisier's theory of the formation of chemical compounds from elements.
Reducing to first principles
- For Van Helmont, earth is not an element because it can be reduced to water.
Inferring the remainder
- Cavendish also described an experiment in which he was able to remove, in modern terminology, both the oxygen and nitrogen gases from a sample of atmospheric air until only a small bubble of unreacted gas was left in the original sample. Using his observations, Cavendish observed that, when he had determined the amounts of phlogisticated air (nitrogen) and dephlogisticated air (oxygen), there remained a volume of gas amounting to 1/120 of the original volume of nitrogen. By careful measurements he was led to conclude that "common air consists of one part of dephlogisticated air [oxygen], mixed with four of phlogisticated [nitrogen]".
Identifying a material (as the same as another material)
- In the 1890s (around 100 years later) two British physicists, William Ramsay and Lord Rayleigh, realised that their newly discovered inert gas, argon, was responsible for Cavendish's problematic residue; he had not made an error. What he had done was perform rigorous quantitative experiments, using standardised instruments and methods, aimed at reproducible results; taken the mean of the result of several experiments; and identified and allowed for sources of error.
- In 1777, Cavendish discovered that air exhaled by mammals is converted to "fixed air" (carbon dioxide), not "phlogisticated air" as predicted by Joseph Priestley.
Showing two materials are the same
- In 1783, José and Fausto Elhuyar found an acid made from wolframite that was identical to tungstic acid.
- By demonstrating that burning diamond and graphite releases the same amount of gas, he established the chemical equivalence of these substances.
Analyzing the remainder
- Around 1735, Swedish chemist Georg Brandt analyzed a dark blue pigment found in copper ore. Brandt demonstrated that the pigment contained a new element, later named cobalt.
- In 1751, a Swedish chemist and pupil of Stahl's named Axel Fredrik Cronstedt, identified an impurity in copper ore as a separate metallic element, which he named nickel.
- Antoine-Laurent de Lavoisier demonstrated with careful measurements that transmutation of water to earth was not possible, but that the sediment observed from boiling water came from the container.
- In addition to studying Priestley's dephlogisticated air, Lavoisier studied more thoroughly the residual air after metals had been calcined. He showed that this residual air supported neither combustion nor respiration.
Material as responsible agent (rather than instrument)
- Few of Epicurus' writings survive and those that do reflect his interest in applying Democritus' theories to assist people in taking responsibility for themselves and for their own happiness—since he held there are no gods around that can help them. He understood gods' role as moral ideals.
- The three metallic principles (sulphur to flammability or combustion, mercury to volatility and stability, and salt to solidity) became the tria prima of the Swiss alchemist Paracelsus. He reasoned that Aristotle's four-element theory appeared in bodies as three principles. Paracelsus saw these principles as fundamental and justified them by recourse to the description of how wood burns in fire. Mercury included the cohesive principle, so that when it left the wood (in smoke) the wood fell apart. Smoke described the volatility (the mercurial principle), the heat-giving flames described flammability (sulphur), and the remnant ash described solidity (salt).
- Mary incorporated lifelike attributes into her descriptions of metal such as bodies, souls, and spirits.
What is loved and hated
- Kore Kosmou: And Isis answer made: Of living things, my son, some are made friends with fire, and some with water, some with air, and some with earth, and some with two or three of these, and some with all. And, on the contrary, again some are made enemies of fire, and some of water, some of earth, and some of air, and some of two of them, and some of three, and some of all. For instance, son, the locust and all flies flee fire; the eagle and the hawk and all high-flying birds flee water; fish, air and earth; the snake avoids the open air.
- Kore Kosmou: Whereas snakes and all creeping things love earth; all swimming things love water; winged things, air, of which they are the citizens; while those that fly still higher love the fire and have the habitat near it. Not that some of the animals as well do not love fire; for instance salamanders, for they even have their homes in it. It is because one or another of the elements doth form their bodies’ outer envelope. Each soul, accordingly, while it is in its body is weighted and constricted by these four.
Removing material-agent
- Boyle performed numerous investigations with an air pump, and noted that the mercury fell as air was pumped out.
- Boyle observed that pumping the air out of a container would extinguish a flame and kill small animals placed inside.
Functions in mixtures
- According to ancient Buddhist atomism, which probably began developing before the 4th century BCE, there are four kinds of atoms, corresponding to the standard elements. Each of these elements has a specific property, such as solidity or motion, and performs a specific function in mixtures, such as providing support or causing growth.
- Mary believed that metals had two different genders and by joining these two genders together a new entity could be made. By joining the different gendered substances together a unity of substances could be obtained.
Reworking the same material in a different form - invariant
- Metalworking in ancient cultures.
- Egyptian weapons made from meteoric iron in about 3000 BC were highly prized as "daggers from Heaven".
Noting which addition of material (reagent, reactant) causes a reaction
- Chemical affinity
- the influential 1923 textbook Thermodynamics and the Free Energy of Chemical Reactions by Gilbert N. Lewis and Merle Randall led to the replacement of the term "affinity" by the term "free energy"
Extracting material by controlling conditions
- Certain metals can be recovered from their ores by simply heating the rocks in a fire: notably tin, lead and (at a higher temperature) copper. This process is known as smelting.
- Techniques for smelting copper and tin from naturally occurring outcroppings of copper ores.
- During the early stages of metallurgy, methods of purification of metals were sought, and gold, known in ancient Egypt as early as 2900 BC, became a precious metal.
- Distilling.
- Removing impurities.
- Taking various steps to remove undesirable contaminants.
- Phosphorus and sulfur may be burnt out of the molten iron.
- The extraction of iron from its ore into a workable metal is much more difficult than copper or tin.
 Ferrous metallurgy
Ferrous metallurgy
- Blast furnace Bloomeries for iron, blowing houses for tin, and smelt mills for lead would be classified as blast furnaces. However, the term has usually been limited to those used for smelting iron ore to produce pig iron.
- Lavoisier passed water through a red-hot iron gun barrel, allowing the oxygen to form an oxide with the iron and the hydrogen to emerge from the end of the pipe.
New materials
Observable distinguishing properties
- Copper is the preferred material for stills because it yields an overall better-tasting spirit. The taste is improved by the chemical reaction between the copper in the still and the sulfur compounds created by the yeast during fermentation.
- Van Helmont is regarded as the founder of pneumatic chemistry, as he was the first to understand that there are gases distinct in kind from atmospheric air and furthermore invented the word "gas". He perceived that his "gas sylvestre" (carbon dioxide) given off by burning charcoal, was the same as that produced by fermenting must, a gas which sometimes renders the air of caves unbreathable.
- Davy also experimented with gases by inhaling them. This experimental procedure nearly proved fatal on several occasions, but led to the discovery of the unusual effects of nitrous oxide, which came to be known as laughing gas.
Measuring densities or ratios
- He then measured the gases' solubility in water and their specific gravity, and noted their combustibility.
- Eudiometer measures volume of a gas mixture under changing physical or chemical conditions.
- With Pierre-Simon Laplace, Lavoisier used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced.
Standardizing measurement of properties - achieving a threshold
- A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually. Hence, a pH indicator is a chemical detector for hydronium ions (H3O+) or hydrogen ions (H+) in the Arrhenius model. Normally, the indicator causes the color of the solution to change depending on the pH.
- Indicators can also show change in other physical properties; for example, olfactory indicators show change in their odor.
Changing properties
- The idea that compounds can have secondary properties that differ from the properties of the elements which are combined to make them became the basis of molecular chemistry.
- The ability of chemical processes to alter the composition of an object without significantly altering its form is the basis of fossil theory via mineralization and the understanding of numerous metallurgical, biological, and geological processes.
Changing phases
- In 1761 Black deduced that the application of heat to ice at its melting point does not cause a rise in temperature of the ice/water mixture, but rather an increase in the amount of water in the mixture.
- Black observed that the application of heat to boiling water does not result in a rise in temperature of a water/steam mixture, but rather an increase in the amount of steam.
Mixing and comparing before and after
- By combining copper and tin, a superior metal could be made, an alloy called bronze.
- A 100,000-year-old ochre-processing workshop was found at Blombos Cave in South Africa. It indicates that early humans had an elementary knowledge of chemistry. Paintings drawn by early humans consisting of early humans mixing animal blood with other liquids found on cave walls also indicate a small knowledge of chemistry.
- Cast iron can be made directly from the molten pig iron or by re-melting pig iron, often along with substantial quantities of iron, steel, limestone, carbon (coke). Depending on the application, carbon and silicon content are adjusted to the desired levels, which may be anywhere from 2–3.5% and 1–3%, respectively.
- By dissolving alkalis in acids, Cavendish produced carbon dioxide, which he collected, along with other gases, in bottles inverted over water or mercury.
Establishing ratios for combination
- The idea that the same elements can be predictably combined in different ratios using different methods to create compounds with radically different properties became the basis of stoichiometry, crystalography, and established studies of chemical synthesis.
- According to the Jabirian version of this theory, metals form in the earth through the mixing of sulfur and mercury. Depending on the quality of the sulfur, different metals are formed, with gold being formed by the most subtle and well-balanced sulfur.
Science of the balance
- A significant part of Jabir's writings were informed by a philosophical theory known as "the science of the balance" (Arabic: ʿilm al-mīzān), which was aimed at reducing all phenomena (including material substances and their elements) to a system of measures and quantitative proportions.
Conservation of mass implies existence
- The law of
 conservation of mass implies that mass can neither be created nor destroyed, although it may be rearranged in space, or the entities associated with it may be changed in form. For example, in chemical reactions, the mass of the chemical components before the reaction is equal to the mass of the components after the reaction. Thus, during any chemical reaction and low-energy thermodynamic processes in an isolated system, the total mass of the reactants, or starting materials, must be equal to the mass of the products.
conservation of mass implies that mass can neither be created nor destroyed, although it may be rearranged in space, or the entities associated with it may be changed in form. For example, in chemical reactions, the mass of the chemical components before the reaction is equal to the mass of the components after the reaction. Thus, during any chemical reaction and low-energy thermodynamic processes in an isolated system, the total mass of the reactants, or starting materials, must be equal to the mass of the products.
- Helmont grew a willow tree and measured the amount of soil, the weight of the tree and the water he added. After five years the plant had gained about 164 lbs (74 kg). Since the amount of soil was nearly the same as it had been when he started his experiment (it lost only 57 grams), he deduced that the tree's weight gain had come entirely from water.
- Antoine-Laurent de Lavoisier burnt phosphorus and sulfur in air, and proved that the products weighed more than the original samples, with the mass gained being lost from the air. Thus, in 1789, he established the Law of Conservation of Mass, which is also called "Lavoisier's Law."
- Analytical balance
Conservation implies latency
- From his observations, Black concluded that the heat applied must have combined with the ice particles and boiling water and become latent.
Manifesting all combinations
- Aristotle: Fire is both hot and dry. Air is both hot and wet. Water is both cold and wet. Earth is both cold and dry.
Controlling the chamber
Collecting device
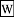 Pneumatic trough The bottle is filled with water, inverted, and placed into the pneumatic trough already containing water. The outlet tube from the gas-generating apparatus is inserted into the opening of the bottle so that gas can bubble up through it, displacing the water within.
Pneumatic trough The bottle is filled with water, inverted, and placed into the pneumatic trough already containing water. The outlet tube from the gas-generating apparatus is inserted into the opening of the bottle so that gas can bubble up through it, displacing the water within.
Collect same material in a different form (such as vapors)
- Kerotakis (Greek: κηροτακίς or κυροτακίς), is a device used to heat substances used in alchemy and to collect vapors.[13] It is an airtight container with a sheet of copper upon its upper side. When working properly, all its joints form a tight vacuum. The use of such sealed containers in the hermetic arts led to the term "hermetically sealed". The kerotakis was said to be a replication of the process of the formation of gold that was occurring in the bowels of the earth.
Identifying irrelevant factors
- Many alchemists included in their methods irrelevant information such as the timing of the tides or the phases of the moon.
Hermetic chamber to capture gases
- Black found that limestone (calcium carbonate) could be heated or treated with acids to yield a gas he called "fixed air." He observed that the fixed air was denser than air and did not support either flame or animal life. Black also found that when bubbled through an aqueous solution of lime (calcium hydroxide), it would precipitate calcium carbonate. He used this phenomenon to illustrate that carbon dioxide is produced by animal respiration and microbial fermentation.
Limiting maximum temperature
- The bain-marie (Mary's bath), which limits the maximum temperature of a container and its contents to the boiling point of a separate liquid: essentially a double boiler. It is extensively used in chemical processes for which a gentle heat is necessary.
Distilling (changing liquid to gas and back)(seperation by selective boiling and condensation)
- An
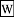 alembic is an alchemical still consisting of two vessels connected by a tube, used for distilling.
alembic is an alchemical still consisting of two vessels connected by a tube, used for distilling.
- A still is an apparatus used to distill liquid mixtures by heating to selectively boil and then cooling to condense the vapor. A still uses the same concepts as a basic distillation apparatus, but on a much larger scale.
- Since ethanol boils at a much lower temperature than water, simple distillation can separate ethanol from water by applying heat to the mixture.
- Distillation is an effective and traditional method of desalination.
- Cryogenic distillation leads to the separation of air into its components – notably oxygen, nitrogen, and argon – for industrial use.
- In the chemical industry, large amounts of crude liquid products of chemical synthesis are distilled to separate them, either from other products, from impurities, or from unreacted starting materials.
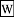 Soxhlet extractor was originally designed for the extraction of a lipid from a solid material. Typically, Soxhlet extraction is used when the desired compound has a limited solubility in a solvent, and the impurity is insoluble in that solvent. It allows for unmonitored and unmanaged operation while efficiently recycling a small amount of solvent to dissolve a larger amount of material.
Soxhlet extractor was originally designed for the extraction of a lipid from a solid material. Typically, Soxhlet extraction is used when the desired compound has a limited solubility in a solvent, and the impurity is insoluble in that solvent. It allows for unmonitored and unmanaged operation while efficiently recycling a small amount of solvent to dissolve a larger amount of material. Fueling a process
- Multiplication is the process in Western alchemy used to increase the potency of the philosopher's stone, elixir or projection powder.
Catalyzing a process
- Projection was the ultimate goal of Western alchemy. Once the Philosopher's stone or powder of projection had been created, the process of projection would be used to transmute a lesser substance into a higher form; often lead into gold. Typically, the process is described as casting a small portion of the Stone into a molten base metal.
Three-cycle
Systematic naming of ingredients, of compounds
- Alchemy had no systematic naming scheme for new compounds, and the language was esoteric and vague to the point that the terminologies meant different things to different people.
Classifying substances (agents)
- The Arabic works attributed to the 8th-century alchemist Jābir ibn Hayyān introduced a systematic classification of chemical substances.
Clarifying social convention
- Democritus wrote that atoms and void are the only things that exist and that all other things are merely said to exist by social convention.
- Lavoisier, together with Louis-Bernard Guyton de Morveau, Claude-Louis Berthollet, and Antoine François de Fourcroy, submitted a new program for the reforms of chemical nomenclature to the Academy in 1787, for there was virtually no rational system of chemical nomenclature at this time. This work, titled Méthode de nomenclature chimique (Method of Chemical Nomenclature, 1787), introduced a new system which was tied inextricably to Lavoisier's new oxygen theory of chemistry. The Classical elements of earth, air, fire, and water were discarded, and instead some 55 substances which could not be decomposed into simpler substances by any known chemical means were provisionally listed as elements.
Apply on a mass scale for social power (for uniformity of materials, measures, instruments so that experiments can be reproduced, can be consistent)
- The history of metallurgy was marked by armies seeking better weaponry. States in Eurasia prospered when they made the superior alloys, which, in turn, made better armor and better weapons.
- While iron is not better suited for tools than bronze (until steel was discovered), iron ore is much more abundant and common than either copper or tin, and therefore more often available locally, with no need to trade for it.
- The amounts of cast iron used for cannon required large scale production.[3] The first cast-iron bridge was built during the 1770s by Abraham Darby III, and is known as The Iron Bridge in Shropshire, England. Cast iron was also used in the construction of buildings.
- The beverage industry was the first to implement a modern distillation apparatus and led the way in developing equipment standards which are now widely accepted in the chemical industry.
- du Pont started a gunpowder mill on the Brandywine River in Delaware. Wanting to make the best powder possible, du Pont was vigilant about the quality of the materials he used.
Clarify what is known and not known. Describe processes.
- Results needed to be reported in a clear language that laid out both what was known and what was unknown.
- Practical attempts to improve the refining of ores and their extraction to smelt metals was an important source of information for early chemists in the 16th century, among them Georg Agricola (1494–1555), who published his great work De re metallica in 1556. His work describes the highly developed and complex processes of mining metal ores, metal extraction and metallurgy of the time. His approach removed the mysticism associated with the subject, creating the practical base upon which others could build. The work describes the many kinds of furnace used to smelt ore, and stimulated interest in minerals and their composition.
Central way
Controlling (enclosing, overseeing) the application of energy to transform a set of inputs to a set of outputs.
- Burning wood
- Boiling water
- Cooking
- Fire for heating
- Fire for lighting
- The Arabic works attributed to the 8th-century alchemist Jābir ibn Hayyān provided instructions for deriving an inorganic compound (sal ammoniac or ammonium chloride) from organic substances (such as plants, blood, and hair) by chemical means.
- A
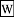 chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products, and shows the direction of the reaction.
chemical equation is the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side with a plus sign between the entities in both the reactants and the products and an arrow that points towards the products, and shows the direction of the reaction.
- Louis Claude Cadet de Gassicourt (24 July 1731 – 17 October 1799) obtained a red liquid by the reaction of potassium acetate with arsenic trioxide. This liquid is known as Cadet's fuming liquid and contains the two compounds cacodyl and cacodyl oxide.
- Many investigators had been experimenting with the combination of Henry Cavendish's inflammable air, which Lavoisier termed hydrogen (Greek for "water-former"), with "dephlogisticated air" (air in the process of combustion, now known to be oxygen) by electrically sparking mixtures of the gases.
- Antoine-Laurent de Lavoisier also discovered that the "inflammable air" discovered by Cavendish - which he termed hydrogen (Greek for water-former) - combined with oxygen to produce a dew, as Priestley had reported, which appeared to be water.
- In cooperation with Laplace, Lavoisier synthesized water by burning jets of hydrogen and oxygen in a bell jar over mercury. The quantitative results were good enough to support the contention that water was not an element, as had been thought for over 2,000 years, but a compound of two gases, hydrogen and oxygen.
Electrolysis
- English chemist Humphry Davy was a pioneer in the field of electrolysis, using Alessandro Volta's voltaic pile to split up common compounds and thus isolate a series of new elements. He went on to electrolyse molten salts and discovered several new metals, especially sodium and potassium, highly reactive elements known as the alkali metals. Potassium, the first metal that was isolated by electrolysis, was discovered in 1807 by Davy, who derived it from caustic potash (KOH).
- Sodium was first isolated by Davy in the same year by passing an electric current through molten sodium hydroxide (NaOH).
- Davy discovered calcium in 1808 by electrolyzing a mixture of lime and mercuric oxide.
- He worked with electrolysis throughout his life and, in 1808, he isolated magnesium, strontium and barium.
- Scheele ultimately obtained oxygen by heating mercuric oxide, silver carbonate, magnesium nitrate, and other nitrate salts.
Analyzing output
- In 1772, the French scientist Antoine Lavoisier used a lens to concentrate the rays of the sun on a diamond in an atmosphere of oxygen, and showed that the only product of the combustion was carbon dioxide, proving that diamond is composed of carbon.
Isolating a material
- José and Fausto Elhuyar succeeded in isolating the metal now known as tungsten by reduction of tungstic acid with charcoal, and they are credited with the discovery of the element.
4 levels
Consider what happens if you keep dividing matter. (As a real experiment or thought experiment).
- Dividing matter generally yields two or more pieces with the same properties.
- Democritus: It is impossible to keep dividing matter for infinity and that matter must therefore be made up of extremely tiny particles.
- Parmenides believed there is no such thing as void, equating it with non-being. This in turn meant that motion is impossible, because there is no void to move into.
- Parmenides wrote all that is must be an indivisible unity, for if it were manifold, then there would have to be a void that could divide it.
- Parmenides stated that the all encompassing Unity is unchanging, for the Unity already encompasses all that is and can be.
- Minima naturalia were theorized by Aristotle as the smallest parts into which a homogeneous natural substance (e.g., flesh, bone, or wood) could be divided and still retain its essential character. Unlike the atomism of Democritus, the Aristotelian "natural minimum" was not conceptualized as physically indivisible. Instead, the concept was rooted in Aristotle's hylomorphic worldview, which held that every physical thing is a compound of matter (Greek hyle) and an immaterial substantial form (Greek morphe) that imparts its essential nature and structure. For instance, a rubber ball for a hylomorphist like Aristotle would be rubber (matter) structured by spherical shape (form). Aristotle's intuition was that there is some smallest size beyond which matter could no longer be structured as flesh, or bone, or wood, or some other such organic substance that for Aristotle, living before the microscope, could be considered homogeneous. For instance, if flesh were divided beyond its natural minimum, what would be left might be a large amount of the element water, and smaller amounts of the other elements. But whatever water or other elements were left, they would no longer have the "nature" of flesh: in hylomorphic terms, they would no longer be matter structured by the form of flesh; instead the remaining water, e.g., would be matter structured by the form of water, not the form of flesh.
- Boyle commented that the finest division of matter where the properties are retained is at the level of corpuscles.
Proportional conditions for chamber
- Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system.
Cause of properties - Correspondence
- Democritus: Human perceptions are caused by atoms. Bitterness is caused by small, angular, jagged atoms passing across the tongue;[9] whereas sweetness is caused by larger, smoother, more rounded atoms passing across the tongue.
- Democritus: The different possible packings and scatterings within the void make up the shifting outlines and bulk of the objects that organisms feel, see, eat, hear, smell, and taste.
- Plato postulated the geometric structure of the simple bodies of the four elements as summarized in the adjacent table. The cube, with its flat base and stability, was assigned to earth; the tetrahedron was assigned to fire because its penetrating points and sharp edges made it mobile. The points and edges of the octahedron and icosahedron were blunter and so these less mobile bodies were assigned to air and water. Since the simple bodies could be decomposed into triangles, and the triangles reassembled into atoms of different elements, Plato's model offered a plausible account of changes among the primary substances.
Reverse explanation by (ratios of) constituents of compounds
- The interpretation of water as a compound explained the inflammable air generated from dissolving metals in acids (hydrogen produced when water decomposes) and the reduction of calces by inflammable air (a combination of gas from calx with oxygen to form water).
- This continuous slow combustion, which they supposed took place in the lungs, enabled the living animal to maintain its body temperature above that of its surroundings, thus accounting for the puzzling phenomenon of animal heat.
- Arrhenius proposed that, even in the absence of an electric current, aqueous solutions of salts contained ions. He thus proposed that chemical reactions in solution were reactions between ions.
- Dalton's law, which describes the relationship between the components in a mixture of gases and the relative pressure each contributes to that of the overall mixture. Discovered in 1801, this concept is also known as Dalton's law of partial pressures.
- Dalton inferred proportions of elements in compounds by taking ratios of the weights of reactants, setting the atomic weight of hydrogen to be identically one. Following Jeremias Benjamin Richter (known for introducing the term stoichiometry), he proposed that chemical elements combine in integral ratios. This is known as the law of multiple proportions or Dalton's law.
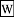 Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated.
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated.
- Berzelius developed the radical theory of chemical combination, which holds that reactions occur as stable groups of atoms called radicals are exchanged between molecules. He believed that salts are compounds formed of acids and bases, and discovered that the anions in acids were attracted to a positive electrode (the anode), whereas the cations in a base were attracted to a negative electrode (the cathode).
Levels of manifestation
- Democritus believed that atoms are too small for human senses to detect, they are infinitely many, they come in infinitely many varieties, and that they have always existed.
- The atomistic theory aimed to remove the "distinction which the Eleatic school drew between the Absolute, or the only real existence, and the world of change around us."
- Parmenides denied the existence of motion, change and void. He believed all existence to be a single, all-encompassing and unchanging mass (a concept known as monism), and that change and motion were mere illusions.
- Parmenides explicitly rejected sensory experience as the path to an understanding of the universe and instead used purely abstract reasoning.
- Democritus believed change was real, and if it was not then at least the illusion had to be explained.
- Democritus supported the concept of void, and stated that the universe is made up of many Parmenidean entities that move around in the void.
- Democritus: The void is infinite and provides the space in which the atoms can pack or scatter differently.
- Democritus: While organisms may feel hot or cold, hot and cold actually have no real existence. They are simply sensations produced in organisms by the different packings and scatterings of the atoms in the void that compose the object that organisms sense as being "hot" or "cold".
6 pairs
Flow through plates in series
- Volta demonstrated in 1794 that when two metals and brine-soaked cloth or cardboard are arranged in a circuit they produce an electric current.
- In 1800, Volta stacked several pairs of alternating copper (or silver) and zinc discs (electrodes) separated by cloth or cardboard soaked in brine (electrolyte) to increase the electrolyte conductivity.[50] When the top and bottom contacts were connected by a wire, an electric current flowed through this voltaic pile and the connecting wire. Thus, Volta is credited with constructing the first electrical battery to produce electricity.
- A Galvanic cell (or voltaic cell) is an electrochemical cell that derives electrical energy from a spontaneous redox reaction taking place within the cell. It generally consists of two different metals connected by a salt bridge, or individual half-cells separated by a porous membrane.
Steps in series. Intermediate steps.
- Smelting iron ore to produce pig iron, an intermediate material used in the production of commercial iron and steel.
- Phosphorus and sulfur may be burnt out of the molten iron, but this also burns out the carbon, which must be replaced.
- Often expressed as a series of color changes or chemical processes, the instructions for creating the philosopher's stone are varied. When expressed in colors, the work may pass through phases of nigredo, albedo, citrinitas, and rubedo.
Establishing cycles.
- The doctrine of five phases. Generating: Wood feeds fire; Fire creates earth (ash); Earth bears metal; Metal collects water; Water nourishes wood.
- The doctrine of five phases. Overcoming: Wood parts earth; Earth absorbs water; Water quenches fire; Fire melts metal; Metal chops wood.
- The doctrine of five phases. There are also two cycles of imbalance, an overacting cycle (乘,cheng) and an insulting cycle (侮,wu).
Removing compounds
- Unwanted and flavor-changing sulfur compounds are chemically removed from the final product resulting in a smoother, better-tasting drink.
Dissolve solute with solvent
- A solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent.
Filtration
- Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass.[citation needed] Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate.[1] Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles (depending on the pore size, filter thickness and biological activity).
Comparing and identifying processes
- Lavoisier and Laplace designed an ice calorimeter apparatus for measuring the amount of heat given off during combustion or respiration. The outer shell of the calorimeter was packed with snow, which melted to maintain a constant temperature of 0 °C around an inner shell filled with ice. By measuring the quantity of carbon dioxide and heat produced by confining a live guinea pig in this apparatus, and by comparing the amount of heat produced when sufficient carbon was burned in the ice calorimeter to produce the same amount of carbon dioxide as that which the guinea pig exhaled, they concluded that respiration was, in fact, a slow combustion process.
- With Pierre-Simon Laplace, Lavoisier used a calorimeter to estimate the heat evolved per unit of carbon dioxide produced. They found the same ratio for a flame and animals, indicating that animals produced energy by a type of combustion.
Outputting fixed ratios of materials
- A certain quantity of electricity during electrolysis or electrodeposition of metals was shown to be associated with certain quantities of chemical elements, and fixed quantities of the elements therefore with each other, in specific ratios.
Reaction tables
- The affinity concept was very closely linked to the visual representation of substances on a table. The first-ever affinity table, which was based on displacement reactions, was published in 1718 by the French chemist Étienne François Geoffroy.
- Crucially, the table was the central graphic tool used to teach chemistry to students and its visual arrangement was often combined with other kinds diagrams. Joseph Black, for example, used the table in combination with chiastic and circlet diagrams to visualise the core principles of chemical affinity.
- In chemical physics and physical chemistry, chemical affinity is the electronic property by which dissimilar chemical species are capable of forming chemical compounds. Chemical affinity can also refer to the tendency of an atom or compound to combine by chemical reaction with atoms or compounds of unlike composition.
- In modern terms, we relate affinity to the phenomenon whereby certain atoms or molecules have the tendency to aggregate or bond.
- George W. Carey states that, "Health depends on a proper amount of iron phosphate Fe3(PO4)2 in the blood, for the molecules of this salt have chemical affinity for oxygen and carry it to all parts of the organism."
Law of multiple proportions (different combinations use the same unit amounts)
- If two elements form more than one compound, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios of small whole numbers.
- For example, Dalton knew that the element carbon forms two oxides by combining with oxygen in different proportions. A fixed mass of carbon, say 100 grams, may react with 133 grams of oxygen to produce one oxide, or with 266 grams of oxygen to produce the other. The ratio of the masses of oxygen that can react with 100 grams of carbon is 266:133 = 2:1, a ratio of small whole numbers. In modern notation the first is CO (carbon monoxide) and the second is CO2 (carbon dioxide).
Law of definite proportions (fixed ratio of element in compound)
- A given chemical compound always contains its component elements in fixed ratio (by mass) and does not depend on its source and method of preparation.
- For example, oxygen makes up about 8/9 of the mass of any sample of pure water, while hydrogen makes up the remaining 1/9 of the mass: the mass of two elements in a compound are always in the same ratio.
- French chemist Joseph Proust proposed the law of definite proportions, which states that elements always combine in small, whole number ratios to form compounds, based on several experiments conducted between 1797 and 1804[56] Along with the law of multiple proportions, the law of definite proportions forms the basis of stoichiometry. The law of definite proportions and constant composition do not prove that atoms exist, but they are difficult to explain without assuming that chemical compounds are formed when atoms combine in constant proportions. * In 1828 Berzelius compiled a table of relative atomic weights, where oxygen was used as a standard, with its weight set at 100, and which included all of the elements known at the time. This work provided evidence in favor of Dalton's atomic theory - that inorganic chemical compounds are composed of atoms combined in whole number amounts. He determined the exact elementary constituents of a large number of compounds; the results strongly supported Proust's Law of Definite Proportions.
Unified theory
Unified framework
- The revolution in chemistry which Lavoisier brought about was a result of a conscious effort to fit all experiments into the framework of a single theory.
Notes
- Analytical chemistry Analytical chemistry studies and uses instruments and methods used to separate, identify, and quantify matter.[1] In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry is the science of obtaining, processing, and communicating information about the composition and structure of matter. In other words, it is the art and science of determining what matter is and how much of it exists.
See with your own eyes what is usually described with equations in a textbook; to experience the rate of a reaction in "real time"; to determine rather than just calculate chemical equilibrium; to observe instead of just predicting the outcome of a chemical reaction.
We will visit chemical equilibrium. We will study the formation of different coordination compounds of nickel, analyze how temperature affects the coordination chemistry of cobalt, and study the solubility equilibrium of several silver compounds. This will be followed by a practical application, the preparation of a black and white Polaroid negative.
We will focus on electrochemistry. Several processes that involve electron transfer reactions, like plating, corrosion, and oxidation of sugars will be examined along with the preparation of a cyanotype print (from the previously prepared negative).
We will study the kinetic mechanism of the reaction between potassium iodide and potassium persulfate, a.k.a. the Iodine Clock Reaction.
We will take a look at a couple of organic compounds with interesting properties, polymers and dyes, by synthesizing Nylon 6,10 (a commercial polymer) and Methyl Orange (a common azo-dye and acid-base indicator).
- https://en.wikipedia.org/wiki/Destructive_distillation
- Fermentation, bronze alloy, fire, Haber-Bosch process, silicon, saponification.
